
CASCADE PAPERS
Autologous Platelet‐Rich Fibrin Matrix as Cell Therapy in the Healing of Lower-Extremity Ulcers (2008) 1
- 12 patients, with 17 VLU, were treated with one to three PRFM applications along with appropriate standard wound care.
- 64.7% of VLUs (66.7% of patients) treated with PRFM closed within the study period of 16 weeks, with an average of two applications per patient.
- The mean time to complete closure in patients with VLU was 7.1 weeks (median 6 weeks).
- No ulcer that achieved complete closure during the16-week study reopened during the evaluation period.
- Data indicated ulcers responding to the PRFM diverge rapidly (by week 3) in their progress to closure compared with ulcers that do not respond.
- No treatment-associated complications or adverse reactions were observed during the 16-week study.
Evaluation of an Autologous Platelet Rich Fibrin Matrix Technology for Diabetic Foot Ulcer Treatment. (2007) 2
- Pilot study with 4 DFU patients with wounds open for 12 weeks to three years, ranging from 5.22 to 11.55 cm2 in area.
- Patients were clinically evaluated weekly and were treated with a PRFM every two weeks, for a maximum of eight weeks, with a four week follow-up.
- 4 of 4 patients met the primary endpoint of 75% healing by 12 weeks, with mean time to complete healing of 9 weeks.
- 3 of 4 patients met the secondary endpoint of 100% healing by 12 weeks.
- No treatment-related serious adverse events were reported.
Cascade Autologous System Platelet-Rich Fibrin Matrix in the Treatment of Chronic Leg Ulcers (2011) 3
- Provides an excellent review of Cascade PRFM technology, prior clinical work, and some of the literature on Cascade included in this list.
Characterization of Autologous Growth Factors in Cascade Platelet Rich Fibrin Matrix (2008) 4
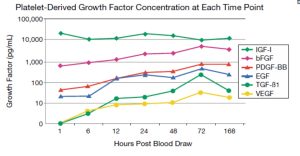
- PRFM was cultured in vitro with media replaced at each time point (“washed-out”), so only the GFs released since the previous time point would be measured.
- Platelets within the PRFM remain functionally viable and continually release all GFs within the PRFM out to 7 days, in vitro, at room temperature.
- The production of five, out of the six, GFs examined exhibited an increasing rate of release out to 72 hours post blood draw. The exception is IGF-I which maintained a relatively constant level.
- [Graph describing GF concentrations]
Flow Cytometric Characterization of CASCADE® Platelet-Rich Fibrin Matrix (PRFM): The Impact of Exogenous Thrombin on Platelet Concentrates (2006) 5
- Demonstrated that platelets isolated from blood using the Cascade system are essentially intact and inactivated.
- In contrast, 5 minute exposure to 100 U/mL (U = International bovine thrombin unit) resulted in >95% activation and >65% degranulation exposed platelets.
A Recently Developed Bifacial Platelet-Rich Fibrin Matrix (2010) 6
- PRFM kit produces a 210-fold higher concentration of platelets when compared to the initial input of whole blood.
- Platelets observed in PRFM mostly have a lenticular shape that is consistent with an inactivated state.
- Growth factors released from the PRFM are active and sustain the proliferative potential of Mesenchymal Stem Cells.
- PRFM has an average elastic modulus of 937 kPa. This is comparable to arterial tissue and represents approximately 50% of the stiffness of intact human skin.
PRFM Improves Wound Angiogenesis via Inducing Endothelial Cell Proliferation (2011) 7
- Demonstrated Cascade platelet viability, in vitro, from 100% to 60% over 7 days.
- A slow and steady release of growth factors (PDGF, TGF, VEGF) from PRFM, leading to increasing concentrations, was observed in vitro.
- PRFM effectively induced endothelial cell proliferation and functional blood vessels in chronic wounds in a delayed porcine model of ischemic wound angiogenesis.
- VEGF released from PRFM was primarily responsible for endothelial mitogenic response.
Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials – Meznerics - 2022 8
- Odds for complete closure were significantly higher in the PRP group than in the control group for all ulcers and subgrouping based on ulcer etiology (DFUs and VLUs).
- Odds for complete closure were significantly higher in VLUs than in DFUs treated with PRP.
- Studies that reported the reduction of wound area showed a significant difference between the post-treatment wound size of the PRP and the control groups.
- Studies that reported healing times showed shorter healing times in the PRP groups than control groups.
- Studies that reported adverse events either recorded no adverse events or no statistically significant difference between the PRP and the control groups.
- The authors conclude that “Platelet-rich plasma is a safe and effective modality to enhance wound healing.”
Platelet-Rich Plasma and Platelet Gel: A Review (2006) 9
- This review provides an excellent review of platelet anatomy and function, platelet actions in hemostasis, platelet physiology in wound healing, and platelet actions in bone healing.
- After injury, platelets: become activated, change shape, spread over the site of injury (platelet aggregation), form a ‘platelet plug,’ release GF (platelet degranulation), and develop a fibrin network to reinforce the platelet plug. Later, leukocytes release cytokines to break down the fibrin network (fibrinolysis).
- The wound healing process has distinctions based on the type and depth of injury. Broadly, wound healing includes hemostasis, inflammation, matrix deposition, collagen synthesis, epithelialization, and tissue remodeling.
- Some key effects of platelet GFs are to stimulate mesenchymal stem cell proliferation, promote the migration of fibroblasts, stimulate collagen production, and induce angiogenesis.
- The review describes which cell types produce which GFs, what cells each GF acts upon in the stages of healing, and the effect the GFs have on the cells they act on.
Growth Factor Release within Liquid and Solid PRF (2022) 10
- Evaluated liquid and solid PRF matrices, from the blood samples of 4 subjects, for levels of 5 GFs (VEGF, EGF, PDGF, TGF, and Matrix Metallopeptidase 9 (MMP-9)) in vitro.
- All 5 growth factors were released in liquid and solid PRF at all 6 time points (1 hour, 7 hours, 1 day, 2 days, 7 days, and 10 days) with peaks at 7 days except EGF.
- EGF reached a consistent level in solid PRF from 7 hours to 7 days in solid PRF versus liquid PRF where it peaked at 7 hours and then declined.
- At all time points, VEGF release was higher in solid PRF than in liquid PRF, whereas PDGF-BB and MMP-9 were higher in liquid PRF than in solid PRF.
- TGF and EGF were lower in solid PRF than liquid PRF within 24 hours. Thereafter, they were higher in solid PRF than liquid PRF.
MEDICAL ACRONYMS & ABBREVIATIONS GUIDE
Below is a reference list of commonly used medical abbreviations found in the cited research to ensure clarity and understanding.
PRP = Platelet Rich Plasma
PRFM = Platelet-Rich Fibrin Membrane
PRF = Platelet-Rich Fibrin
DFU = Diabetic Foot Ulcer
VLU = Venous Leg Ulcer
RCT = Randomized Clinical Trial
GF = Growth Factors
PDGF = Platelet Derived Growth Factor
TGF = Transforming Growth Factor
VEGF = Vascular Endothelial Growth Factor
IGF = Insulin-Like Growth Factor
EGF = Epidermal Growth Factor
bFGF = Basic Fibroblast Growth Factor

