FAQ'S
Wound healing is a complex process involving a series of coordinated events that aim to repair damaged tissue. This process is generally classified into four overlapping stages:
- Hemostasis: Immediately after injury, platelets activate, aggregate at the injury site, form a clot to control bleeding, and release growth factors.
- Inflammatory Phase: The release of inflammatory mediators attracts immune cells, such as neutrophils and macrophages, to clear debris and fight potential infection.
- Proliferative Phase: This stage focuses on rebuilding new tissue to fill the wound. Fibroblasts, key cells in this phase, produce collagen and other extracellular matrix components, providing structural integrity to the wound. New blood vessels form (angiogenesis) to supply oxygen and nutrients to the regenerating tissue. Epithelial cells migrate from the wound edges, covering the surface and restoring the skin barrier.
- Remodeling Phase: The final stage involves the maturation and reorganization of the newly formed tissue. Collagen fibers realign, increasing the wound’s tensile strength.
Platelet-rich plasma (PRP) is a concentrated preparation of platelets derived from a patient’s own blood. It contains a higher concentration of platelets and growth factors than whole blood. When applied to a wound, PRP releases a potent cocktail of growth factors, including PDGF, TGF-β, VEGF, EGF, IGF-I and FGF, which stimulate and accelerate the various stages of wound healing.
Normal platelet counts in blood range between 150,000/μl to 350,000/μl and average about 200,000/μl. PRP has been shown to be effective in soft tissue healing at 1,000,000 platelets/μl.
- PDGF is a potent chemoattractant for cells involved in wound healing and stimulates the proliferation of fibroblasts, enabling tissue regeneration and repair.
- TGF-β stimulates the production of extracellular matrix proteins, including collagen, promoting wound closure and tissue remodeling.
- VEGF stimulates angiogenesis, the formation of new blood vessels, providing essential oxygen and nutrients for wound healing.
- EGF promotes the proliferation of fibroblasts, endothelial cells, and keratinocytes, accelerating wound closure and re-epithelialization.
- IGF-I stimulates the growth and differentiation of multiple cell types, including fibroblasts and osteoblasts, enhancing tissue repair and regeneration.
- FGF is a potent mitogen for fibroblasts, promoting the formation of granulation tissue and facilitating wound contraction.
Autologous PRP, which is derived from the patient’s own blood, eliminates the risk of immune rejection and disease transmission. It offers a safe and biocompatible approach to wound healing.
CASCADE® is the only solid PRP formulation that does not contain either exogenous thrombin or neutrophils. This makes it uniquely suited for wound healing applications.
A solid fibrin membrane provides a natural scaffold for cell migration, proliferation, and differentiation, creating a favorable environment for tissue regeneration. Unlike liquid PRP, where its platelets and growth factors are quickly absorbed and dispersed from the wound within 24 hours or less, the fibrin network helps trap and concentrate platelets and their growth factors at the wound site, maximizing their impact on the healing process. CASCADE® PRFM has mechanical strength similar to arterial tissue, so it can easily be fenestrated, handled with forceps, and sutured or packed into wounds.
The CASCADE® preparation process does not require exogenous thrombin, a clotting agent commonly used in other PRP systems. Exogenous thrombin carries a potential risk of antibody formation and Factor V deficiency. Exogenous thrombin causes immediate platelet activation and release of growth factors.
The CASCADE® Autologous Platelet System uses the body’s own (endogenous) thrombin and calcium chloride, in the green PRFM bottle, to catalyze the conversion of fibrinogen into the fibrin in the PRFM. By avoiding exogenous thrombin, CASCADE® offers a safe and natural approach to PRFM preparation with sustained growth factor release for 7-10 days.
Neutrophils can produce Neutrophil Extracellular Traps (NETs), web-like extracellular structures made from DNA released from the cell’s interior. While initially recognized as a defense mechanism against pathogens, NETs can hinder tissue regeneration by releasing inflammatory cytokines and enzymes that drive pro-inflammatory and catabolic effects, damaging healthy tissue. To mitigate this, the CASCADE® leukocyte-poor preparation minimizes neutrophil presence, reducing excessive inflammation and fostering a more balanced healing environment.
Platelets play a crucial role in shifting macrophages from a pro-inflammatory (M1) to a pro-healing (M2) state in chronic wounds by releasing key growth factors and signaling molecules.
Macrophages can be polarized into two phenotypes: the classical inflammatory type (M1), that helps fight infection, and the anti-inflammatory type (M2) that helps heal wounds by suppressing inflammation, initiating angiogenesis, and driving wounds towards the repair and remodeling phases. The composition of macrophage phenotypes is shifted towards the M1 inflammatory type in chronic wounds, and the restoration of balance toward the M2 phenotype is beneficial in the later stages of wound healing.Platelet-conditioned media has been shown to induce the anti-inflammatory phenotype in macrophages.
CASCADE® comes with everything needed for point of care preparation of PRFM, including:
- Tourniquet for venipuncture
- Butterfly needle for venipuncture
- Tube Holder
- 2 PRP isolation tubes
- PRP transfer device
- PRFM formation bottle
Centrifuge not included.
Follow the image below for step-by-step instructions to create solid CASCADE® PRFM. 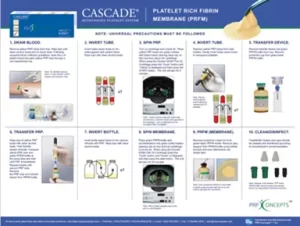
CASCADE® PRFM can be easily handled with forceps.
CASCADE® PRFM has been found to have an average elastic modulus of 937 kPa. That level of mechanical strength is comparable to arterial tissue, which represents approximately 50% of the stiffness of intact human skin.
Prior clinical work has followed this procedure: Freshly prepared membrane was fenestrated using sterile forceps and scissors to introduce uniform slits, approximately 3mm long, to allow drainage of wound exudate, increase moisture and air exchange, and improve intimate application to the wound bed. The membrane was then trimmed to conform to the wound outline and applied with a 0.5cm overlap of the wound margin onto the peri-wound area.
A non-adherent contact layer should be applied directly to the PRFM. The choice of dressings and bandages should align with standard wound care protocols, based on the specific wound type being treated.
Yes! Use CASCADE® alongside the standard of care appropriate for the specific type of wound being treated.
Yes! While research shows that platelet count generally decreases with age, the CASCADE® PRFM technology amplifies the concentration of platelets by 210-fold to the baseline blood level. This substantial increase helps compensate for age-related declines, ensuring a high concentration of growth factors crucial for healing.
CASCADE® has been demonstrated to work effectively in older adults, even in challenging cases involving chronic wounds. A study conducted at the Palo Alto VA outpatient wound clinic showed that CASCADE® PRFM achieved 100% healing in 86% of patients, ages ranging from 58 to 78, with an average healing time of 7.2 weeks. Healed wound sites did not re-ulcerate as patients were followed for 2-5 months after healing.
Gosch C. Zeichner A. Carroll R. Bois J. Evaluation of an autologous platelet rich fibrin matrix technology for diabetic foot ulcer treatment. Wound Rep Regen. 2007;15:A38.90. Abstract.
CASCADE® is contraindicated in patients with tumors, metastatic disease, active infection, thrombocytopenia (platelet count below 150,000 platelets per microliter), or pregnancy (Bava & Barber, 2011).
Additionally, potential candidates for PRP treatment should undergo a pretreatment hematologic evaluation to rule out coagulopathies and platelet function disorders. Patients with anemia, thrombocytopenia, hemodynamic instability, severe hypovolemia, unstable angina, sepsis, or those undergoing anticoagulant or fibrinolytic therapy may not be suitable for PRP treatment (Jain & Gulati, 2016).
- PRP = Platelet-Rich Plasma
- PRFM = Platelet-Rich Fibrin Membrane
- PRF = Platelet-Rich Fibrin
- DFU = Diabetic Foot Ulcer
- VLU = Venous Leg Ulcer
- RCT = Randomized Clinical Trial
- GF = Growth Factors
- PDGF = Platelet-Derived Growth Factor
- TGF = Transforming Growth Factor
- VEGF = Vascular Endothelial Growth Factor
- IGF = Insulin-Like Growth Factor
- EGF = Epidermal Growth Factor
- bFGF = Basic Fibroblast Growth Factor

